Explain What Makes One Atom Different From Another
Electrons - negatively charged. An element is a substance in which all of the atoms have the same atomic proton number.

Bill Nye Atoms Video Guide Sheet Teaching Chemistry Persuasive Writing Prompts Earth Science Lessons
For instance the oxygen.

. What does the number represent in the isotope. Typically these elements have the same number of negatively charged electrons orbiting. This is given as the atomic number of the element on the periodic table.
Protons and neutrons form the nucleus of an atom and a cloud of electrons orbits the nucleus. 9 Atoms of different elements with different mass numbers but same number of neutrons 10 Atoms of different elements having same atomic numbers DOWN 2 Atoms of same element having different mass number 3 Number of protons in an atoms 4 Proposed planetary model 6 Fundamental particle with no charge 7 Number of protons and neutrons. They differ by the size of nucleus as volume of nucleus is directly proportional to the mass number of the atom.
Its what makes one element different from another. The number of protons in the nucleus. What distinguishes the atoms of one element from the atoms of another.
By definition an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Molecules are groups of two or more atoms that are chemically bonded. What makes one atom different from another.
An atom is the smallest particle of an element that retains the properties of that element. What makes an atom of gold different from an atom of iron is the number of protons neutrons and electrons inside it. What makes one element different from another is the number of protons in the nucleus of its atoms.
The number of neutrons can be equal or different. An atom is considered to be electrically neutral if it has an equal number of protons and electrons. Ions usually form when electrons are transferred from one atom to another.
The number of protons in an atom is. In this notation the number is the mass number the number of protons neutrons. This is the primary difference between an atom and element.
The number of protons in an atom is the defining feature of an atom. If the number of neutrons is different from one atom to another we name them as isotopes of the chemical element. Therefore to be precise atoms are the smallest part or amounts of elements.
The number of neutrons can vary to produce isotopes which are atoms of the same element that have different numbers of neutrons. Cut apart a single atom of iron and you will find 26 protons and 30 neutrons clumped together in the nucleus. Atoms are made up of three subatomic particles.
Atoms are the smallest unit of matter that cant be broken down chemically. An atom refers to the single one an element is the type of atoms it is. Discover the different parts of an atom such as the nucleus proton neutron and electrons.
An atom is the smallest component of an element containing neutrons protons and electrons and makes up everything around us. The obvious difference described in more detail at the link below is the different number of neutrons in the isotope that make it different from the original element. If an atom has a different number of electrons and protons it is called an ion.
Think of this as similar to the way the. Neutrons - with no charge. An atom is extremely small and its size is around 100 pm.
If an element comes from steel it means it will contain steel atoms only. Click to see full answer. Covalent bonds are generally very _____.
Protons - positively charged. The number of protons determines an elements atomic number Z and distinguishes one element from another. Two different atoms will have different size of atom and nucleus.
An atom can be an ion but not all ions are atoms. The mass of atoms centres to. The protons and neutrons are in the atomic nucleus of the atom while the electrons orbit the atomic nucleus.
The number of protons are different. - Answers Primarily the number of protons in the atoms nucleus which is the same as the number of electrons it has. Shared pairs of electrons fill the _____ energy levels of bonded atoms.
What makes atoms of one element different from the atoms of another element. An important principle to know is electrons may be transferred from one atom to another or even shared between atoms allowing atoms to bind together. To be simpler and in most cases elements are made from only one type of atom.
Also explore atomic bonding the way atoms join together to form molecules. Protons are what make an element that particular element. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.
It is also defined as a substance which cannot be broken down by chemical means Answer link. You can have a single atom and a single element but a single atom is like a dot whereas a single element could be more than one atom but all of the same element. For example carbons atomic number Z is 6 because it has 6 protons.
The number of protons in the nucleus which is generally equal to the number of orbiting electrons is what distinguishes atoms of one element from atoms of another element. And they also differ by the wavelength of light produced by them when an electron jumps from higher energy state to lower energy state. What makes each element different is the number of positively charged protons in the nucleus of the atom.
Atoms are the simplest unit of a matter. They contain the same number of protons as electrons. Secondarily in the number of.
These subatomic particles include. Like everything else atoms have a few different things floating around inside of them. The subatomic particle that makes atoms of different elements different from each other is the proton.
An atom is the smallest repeating unit that makes up all matter. Structure of an Atom.
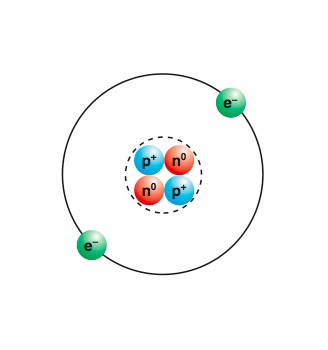
Atoms Molecules And Compounds Manoa Hawaii Edu Exploringourfluidearth

Atoms And The Periodic Table Activities Game For Learning Atomic Structure Middle School Science Classroom Science Chemistry Teaching Chemistry
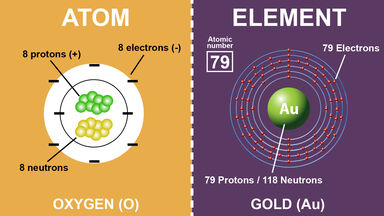
Difference Between Atoms And Elements With Examples

Atoms And Elements Doodle Note Science Doodle Notes Science Doodles Doodle Notes Science Doodle Notes

Comments
Post a Comment